Wrought iron
|
Ferrite (α-iron, δ-iron) |
| Steel classes |
|
Crucible steel
Alloy steel (contains non-carbon elements)
|
| Other iron-based materials |
|
Cast iron (>2.1% carbon)
Wrought iron (contains slag) |

Wrought iron is an iron alloy with a very low carbon content, in comparison to steel, and has fibrous inclusions, known as slag. This is what gives it a "grain" resembling wood, which is visible when it is etched or bent to the point of failure. Wrought iron is tough, malleable, ductile and easily welded. Historically, it was known as "commercially pure iron",[1][2] however it no longer qualifies because current standards for commercially pure iron require a carbon content of less than 0.008 wt%.[3][4]
Before the development of effective methods of steelmaking and the availability of large quantities of steel, wrought iron was the most common form of malleable iron. A modest amount of wrought iron was used as a raw material for manufacturing of steel, which was mainly to produce swords, cutlery and other blades. Demand for wrought iron reached its peak in the 1860s with the adaptation of ironclad warships and railways, but then declined as mild steel became more available.
Before they came to be made of mild steel, items produced from wrought iron included rivets, nails, chains, railway couplings, water and steam pipes, nuts, bolts, horseshoes, handrails, straps for timber roof trusses, and ornamental ironwork.
Wrought iron is no longer produced on a commercial scale. Many products described as wrought iron, such as guard rails, garden furniture[5] and gates, are made of mild steel.[6] They retain that description because they were formerly made of wrought iron or have the appearance of wrought iron. True wrought iron is required for the authentic conservation of historic structures.
Contents |
Terminology
Wrought iron is so named because it is worked from a bloom of porous iron mixed with slag and other impurities. The word "wrought" is an archaic past tense form of the verb "to work". "Wrought iron" literally means "worked iron".
Wrought iron is a general term for the commodity, but is also used more specifically for finished iron goods, as manufactured by a blacksmith or other smith. It was used in this narrower sense in British Customs records, such manufactured iron being subject to a higher rate of duty than what might be called "unwrought" iron.
In the 17th, 18th and 19th centuries, wrought iron went by a wide variety of terms according to its form, origin, or quality.
Form
- Bar iron—iron in bars, which are the usual product of the finery forge, but not necessarily made by that process. These might be square or flat, and flat bars might be narrow or broad.
- Rod iron—cut from flat bar iron in a slitting mill to provide the raw material for nails.
- Hoop iron—suitable for the hoops of barrels, apparently made by passing rod iron through flat rolls.
- Plate iron—sheets of iron suitable for use as boiler plate.
- Blackplate—sheets of iron, perhaps thinner than plate iron, from the black rolling stage of tinplate production.
- Voyage iron—narrow flat bar iron, made or cut into bars of a particular weight, a commodity for sale in Africa for the Atlantic slave trade. The number of bars per ton gradually increased from 70 per ton in the 1660s to 75–80 per ton in 1685 and "near 92 to the ton" in 1731.[7]
Origin
- Charcoal Iron — Until the end of the eighteenth century, wrought iron was made using charcoal, by the bloomery process, in a finery forge or from the industrial revolution in a Lancashire hearth. The resulting metal was highly variable, both in chemistry and slag content.
- Puddled Iron — By the late eighteenth century there was a demand for wrought iron to be refined with coal as fuel. This resulted in ‘puddled iron’. The iron was kept separate from the fire in a reverberatory furnace to prevent harmful sulphur and phosphorus from entering the finished iron. Puddled iron, although also variable in its properties, was generally more consistent than the earlier irons, and the method lent itself to the production of far greater quantities. By 1876, annual production of puddled iron in the UK alone was over 4 million tons.
- Oregrounds iron—a particularly pure grade of bar iron made ultimately from iron ore from the Dannemora mine in Sweden. Its most important use was as the raw material for the cementation process of steelmaking.
- Danks iron — originally iron imported to Great Britain from Danzig (now Gdansk), but in the 18th century more probably the kind of iron (from eastern Sweden) that once came from Danzig.
- Forest iron — iron from the Forest of Dean, where haematite ore enabled tough iron to be produced.
- Lukes iron — iron imported from Liège, whose Dutch name is "Luik."[8]
- Ames iron or amys iron — another variety of iron imported to England from northern Europe. Its origin has been suggested to be Amiens, but it seems to have been imported from Flanders in the 15th century and Holland later, suggesting an origin in the Rhine valley. Its origins remain controversial.[8]
- Botolf iron or Boutall iron — from Butow (Pommerania) or Beuthen (Silesia).[8]
- Sable iron (or Old Sable) — iron bearing the mark (a sable) of the Demidov family of Russian ironmasters, one of the better brands of Russian iron.[9]
Quality
- Tough iron
- Tough iron, also spelled "tuf", is not brittle and strong enough to be used for tools.
- Blend iron
- Blend iron is made using a mixture of different types of pig iron.
- Best iron
- Best iron is iron that had gone through several stages of piling and rolling, might reach the stage of being best iron (in the 19th century).
- Marked bar iron
- This is iron made by members of the Marked Bar Association and marked with the maker's brand mark as a sign of its quality.[10]
Defects
Wrought iron is redshort if it contains sulfur in excess quantity. It has sufficient tenacity when cold, but cracks when bent or finished at a red heat. It is therefore useless for welding or forging.
Coldshort iron, also known as coldshear, colshire or bloodshot, contains excessive phosphorus. It is very brittle when it is cold. It cracks if bent. It may, however, be worked at high temperature. Historically, coldshort iron was considered good enough for nails.
Nevertheless, phosphorus is not necessarily detrimental to iron. Ancient Indian smiths did not add lime to their furnaces; the absence of CaO in the slag, and the deliberate use of wood with high phosphorus content during the smelting, induces a higher P content (> 0.1%, average 0.25%) than in modern iron. There is more phosphorus as solid solution throughout the metal than in the slags (one analysis gives 0.10% in the slags for .18% in the iron itself, for a total P content of 0.28% in the metal). This high P content and particular repartition are essential factors in the formation of a passive protective film of “misawite” (d-FeOOH), an amorphous iron oxyhydroxide that forms a barrier by adhering next to the interface between metal and rust. From this technology recently rediscovered by metallurgists at IIT Kanpur through the study of the Iron Pillar of Delhi, rust-proof iron is at the last stages of being commercialized. This 1600 years-old rust-proof pillar is also of a remarkable strength, having withstood the impact of a cannon ball in the 18th century. Copper has a similar effect as phosphate regarding the formation of a passive protection film.[11][12][13] Furthermore, the presence of phosphorus (without carbon) produces a ductile iron suitable for wire drawing, for piano wire.[14]
History
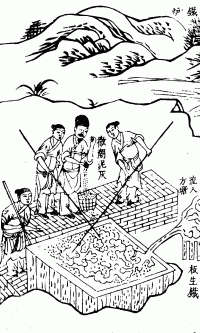
Wrought iron has been used for many centuries, and is the "iron" that is referred to throughout western history. The other form of iron, cast iron, was not introduced into Western Europe until the 15th century; even then, due to its brittleness, it could only be used for a limited number of purposes. Throughout much of the Middle Ages iron was produced by the direct reduction of ore in manually operated bloomeries, although waterpower had begun to be employed by 1104.[15]
The raw material produced by all indirect processes is pig iron. It has a high carbon content and as a consequence it is brittle and could not be used to make hardware. The osmond process was the first of the indirect processes, developed by 1203, but bloomery production continued in many places. The process depended on the development of the blast furnace, of which medieval examples have been discovered at Lapphyttan, Sweden and in Germany.
The bloomery and osmond processes were gradually replaced from the 15th century by finery processes, of which there were two versions, the German and Walloon. They were in turn replaced from the late 18th century by puddling, with certain variants such as the Swedish Lancashire process. These too are now obsolete, and wrought iron is no longer manufactured commercially.
Bloomery process
Wrought iron was originally produced by a variety of smelting processes, all described today as bloomeries. Different forms of bloomery were used at different places and times. The bloomery was charged with charcoal and iron ore and then lit. Air was blown in through a tuyere to heat the bloomery to a temperature somewhat below the melting point of iron. In the course of the smelt, slag would melt and run out, and carbon monoxide from the charcoal would reduce the ore to iron, which formed a spongy mass. The iron remained in the solid state. If the bloomery was allowed to become hot enough to melt the iron, carbon would dissolve into it and form pig or cast iron, but that was not the intention.[16]
After smelting was complete, the bloom was removed, and the process could then be started again. It was thus a batch process, rather than a continuous one. The spongy mass contained iron and also silicate (slag) from the ore; this was iron bloom from which the technique got its name. The bloom had to be forged mechanically to consolidate it and shape it into a bar, expelling slag in the process.[16]
During the Middle Ages, water-power was applied to the process, probably initially for powering bellows, and only later to hammers for forging the blooms. However, while it is certain that water-power was used, the details of this remain uncertain.[17] This was the culmination of the direct process of ironmaking. It survived in Spain and southern France as Catalan Forges to the mid 19th century, in Austria as the stuckofen to 1775,[18] and near Garstang in England until about 1770;[19] it was still in use with hot blast in New York State in the 1880s.[20]
Osmond process
Osmond iron consisted of balls of wrought iron, produced by melting pig iron and catching the droplets on a staff, which was spun in front of a blast of air so as to expose as much of it as possible to the air and oxidise its carbon content.[21] The resultant ball was often forged into bar iron in a hammer mill.
Finery process
In the 15th century, the blast furnace spread into what is now Belgium and was improved. From there, it spread via the Pays de Bray on the boundary of Normandy and then to the Weald in England. With it, the finery forge spread. These remelted the pig iron and (in effect) burnt out the carbon, producing a bloom, which was then forged into a bar iron. If rod iron was required, a slitting mill was used.
The finery process existed in two slightly different forms. In Great Britain, France, and parts of Sweden, only the Walloon process was used. This employed two different hearths, a finery hearth for fining the iron and a chafery hearth for reheating it in the course of drawing the bloom out into a bar. The finery always burnt charcoal, but the chafery could be fired with mineral coal, since its impurities would not harm the iron when it was in the solid state. On the other hand, the German process, used in Germany, Russia, and most of Sweden used a single hearth for all stages.[22]
The introduction of coke for use in the blast furnace by Abraham Darby in 1709 (or perhaps others a little earlier) initially had little effect on wrought iron production. Only in the 1750s was coke pig iron used on any significant scale as the feedstock of finery forges. However, charcoal continued to be the fuel for the finery.
Potting and stamping
From the late 1750s, ironmasters began to develop processes for making bar iron without charcoal. There were a number of patented processes for this, which are referred to today as potting and stamping. The earliest were developed by John Wood of Wednesbury and his brother Charles Wood of Low Mill at Egremont, patented in 1763.[23] Another was developed for the Coalbrookdale Company by the Cranage brothers.[24] Another important one was that of John Wright and Joseph Jesson of West Bromwich.[25]
Puddling process
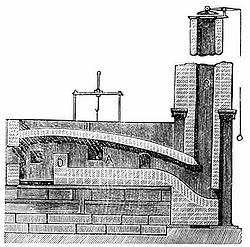
A number of processes for making wrought iron without charcoal were devised as the Industrial Revolution began during the latter half of the 18th century. The most successful of these was puddling, using a puddling furnace (a variety of the reverberatory furnace). This was invented by Henry Cort in 1784.[26] It was later improved by others including Joseph Hall. In this type of furnace, the metal does not come into contact with the fuel, and so is not contaminated by impurities in it. The flame from the fire is reverberated or sent back down onto the metal on the fire bridge of the furnace.
Unless the raw material used is white cast iron, the pig iron or other raw material first had to be refined into refined iron or finers metal. This would be done in a refinery where raw coal is used to remove silicon and convert carbon from a graphitic form to a combined form.
This metal was placed into the hearth of the puddling furnace where it was melted. The hearth was lined with oxidizing agents such as haematite and iron oxide.[27] This mixture is subjected to a strong current of air and stirred with long bars, called puddling bars or rabbles,[28][29] through working doors.[30] The air, stirring, and "boiling" action of the metal help the oxidizing agents to oxidize the impurities and carbon out of the pig iron to their maximum capability. As the impurities oxidize, the retaining material solidifies into spongy wrought iron balls, called puddle balls.[27]
Shingling
There is still some slag left in the puddle balls, so while they are still hot they must be shingled[31] to remove the remaining slag and cinder.[27][31] It may be achieved by forging the balls under a power hammer, or by squeezing the bloom in a machine. The material obtained at the end of shingling is known as bloom and it is still red-hot.[31] The blooms are not useful in this form, so they must be rolled into a final product.
Sometimes European ironworks would skip this step completely and roll the puddle balls. The only drawback to this is that the edges of the rough bars are not as well compressed. When the rough bar is reheated, the edges may separate and be lost into the furnace.[31]
Rolling
The bloom is passed through grooved rollers and flat bars were produced. These bars of wrought iron were of poor quality, called muck bars[31][32] or puddle bars.[27] To improve the quality of wrought iron, these bars were cut up, piled and tied together by wires, a process known as faggoting or piling.[31] They were then reheated and rolled again in merchant rolls. This process may be repeated several times to get wrought iron of desired quality. Wrought iron that has been rolled multiple times is called merchant bar or merchant iron.[29][33]
Lancashire process
The advantage of puddling was that it used coal, not charcoal as fuel. However this was little advantage in Sweden, which lacks coal. Gustaf Ekman observed charcoal fineries at Ulverstone, which were quite different from any in Sweden. After his return to Sweden in the 1830s, he experimented and developed a process similar to puddling but using forewood and charcoal, which was widely adopted in the Bergslagen in the following decades.[34]
The Aston process
In 1925, James Aston of the United States developed a process for manufacturing wrought iron quickly and economically. It involves taking molten steel from a Bessemer converter and pouring it into cooler liquid slag. The temperature of the steel is about 1500 °C and the liquid slag is maintained at approximately 1200 °C. The molten steel contains a large amount of dissolved gases so when the liquid steel hits the cooler surfaces of the liquid slag the gases are liberated. The molten steel then freezes to yield a spongy mass having a temperature of about 1370 °C.[27] This spongy mass must then be finished by being shingled and rolled as described under puddling (above). Three to four tons can be converted per batch with this method.[27]
The end of wrought iron
In the 1960s the price of steel production was dropping due to recycling and even using the Aston process wrought iron production was a labor-intensive process. It has been estimated that the production of wrought iron costs approximately twice as much as the production of low carbon steel.[6] In the United States the last plant closed in 1969.[6] The last in Great Britain (and the world) was the Atlas Ironworks of Thomas Walmley Ltd in Bolton, which closed in 1973. Its equipment, of a type dating from the 1860s, was moved to the Blists Hill site of Ironbridge Gorge Museum for preservation.[35] Some wrought iron is still being produced for heritage restoration purposes, but only by recycling scrap.
Properties

The slag inclusions in wrought iron give it properties not found in other forms of ferrous metal. There are approximately 250,000 inclusions per square inch.[6] A fresh fracture shows a clear bluish color with a high silky luster and fibrous appearance.
Wrought iron lacks the carbon content necessary for hardening through heat treatment, but in areas where steel was uncommon or unknown, tools were sometimes cold-worked (hence cold iron) in order to harden them. An advantage of its low carbon content is its excellent weldability.[6] Furthermore, sheet wrought iron cannot bend as much as steel sheet metal (when cold worked).[36][37][38] Wrought iron can be cast, however there is no engineering advantage as compared to cast iron; cast iron is much easier to produce and thus cheaper, so it is exclusively chosen over wrought iron.[39][40]
Due to the variations in iron ore origin and iron manufacture, wrought iron can be inferior or superior in corrosion resistance compared to other iron alloys.[6][41][42][43] There are many mechanisms behind this corrosion resistance. Chilton and Evans found that nickel enrichment bands reduce corrosion.[44] They also found that in puddled and forged and piled the working over of the iron spread out copper, nickel and tin impurities, which produce electrochemical conditions that slow down corrosion.[42] The slag inclusions have been shown to disperse corrosion in to an even film to resist pitting.[6] Another study has shown that slag inclusions are pathways to corrosion.[45] Other studies show that sulfur impurities in the wrought iron decrease corrosion resistance,[43] but phosphorus increase corrosion resistance.[46] Environments with a high concentration of chlorine ions also decreases wrought iron's corrosion resistance.[43]
Wrought iron has a rough surface so it can hold platings and coatings better. For instance, a galvanic zinc finish is approximately 25–40% thicker than the same finish on steel.[6]
In Table 1, the chemical composition of wrought iron is compared to that of pig iron and carbon steel. Although it appears that wrought iron and plain carbon steel have similar chemical compositions, this is deceiving. Most of the manganese, sulfur, phosphorus, and silicon are incorporated into the slag fibers present in the wrought iron, so wrought iron really is purer than plain carbon steel.[31]
| Material | Iron | Carbon | Manganese | Sulfur | Phosphorus | Silicon |
|---|---|---|---|---|---|---|
| Pig iron | 91–94 | 3.5–4.5 | 0.5–2.5 | 0.018–0.1 | 0.03–0.1 | 0.25–3.5 |
| Carbon steel | 98.1–99.5 | 0.07–1.3 | 0.3–1.0 | 0.02–0.06 | 0.002–0.1 | 0.005–0.5 |
| Wrought iron | 99–99.8 | 0.05–0.25 | 0.01–0.1 | 0.02–0.1 | 0.05–0.2 | 0.02–0.2 |
| All units are percent weight | ||||||
| Property | Value |
|---|---|
| Ultimate tensile strength [psi (MPa)][47] | 34,000–54,000 (234–372) |
| Ultimate compression strength [psi (MPa)][47] | 34,000–54,000 (234–372) |
| Ultimate shear strength [psi (MPa)][47] | 28,000–45,000 (193–310) |
| Yield point [psi (MPa)][47] | 23,000–32,000 (159–221) |
| Modulus of elasticity (in tension) [psi (MPa)][47] | 28,000,000 (193,100) |
| Melting point [°F (°C)][48] | 2,800 (1,540) |
| Specific gravity | 7.6–7.9[49] |
| 7.5–7.8[50] |
Amongst its other properties, wrought iron becomes soft at red heat, and can be easily forged and forge welded.[51] It can be used to form temporary magnets, but cannot be magnetized permanently,[52][53] and is ductile, malleable and tough.[31]
Applications
One application is furniture. It has a long history, dating back to Roman times. There are Thirteenth century wrought iron gates in Westminster Abbey in London, but the Seventeenth century, and the reign of William and Mary in Great Britain, appears to have brought its popularity to a peak. However the coming of cast iron and cheaper steel caused a gradual decline in wrought iron manufacture, with the last wrought ironworks in Britain closing in 1974.[54]
It is also used to make home decor items such as baker's racks, wine racks, pot racks, etageres, table bases, desks, gates, beds, candle holders, curtain rods, bars and bar stools.
See also
- Ornamental metal
- Semi-steel casting
References
Notes
- ↑ Imhoff, Wallace G. (1917), "Puddle Cinder as a Blast Furnace Iron Ore", Journal of the Cleveland Engineering Society 9 (621.76): 332, http://books.google.com/books?id=OA_OAAAAMAAJ.
- ↑ Scoffern, John (1869), The useful metals and their alloys (5th ed.), Houlston & Wright, p. 6, http://books.google.com/books?id=4Ik1AAAAMAAJ.
- ↑ McArthur, Hugh; Spalding, Duncan (2004), Engineering materials science: properties, uses, degradation and remediation, Horwood Publishing, p. 338, ISBN 9781898563112, http://books.google.com/books?id=7dwAaOqp69wC.
- ↑ Campbell, Flake C. (2008), Elements of Metallurgy and Engineering Alloys, ASM International, p. 154, ISBN 9780871708670, http://books.google.com/books?id=6VdROgeQ5M8C.
- ↑ "Wrought Iron: A Patio Furniture dream". cnet reviews. http://www.patioset.com/Wrought_Iron_Patio_Sets/. Retrieved 2009-09-29.
- ↑ 6.0 6.1 6.2 6.3 6.4 6.5 6.6 6.7 Daniel, Todd (May 3, 1997), Clearing the Confusion Over Wrought Iron, http://www.artmetal.com/project/NOMMA/WROUGHT.HTM, retrieved 2008-01-05
- ↑ C. Evans and G. Rydén, Baltic Iron in the Atlantic World in the Eighteenth Century (Brill, Boston, Mass. 2007), 163–73.
- ↑ 8.0 8.1 8.2 W. R. Childs, "England's Iron trade in the Fifteenth Century" Economic History Review 2nd ser. (1981), 25–47.
- ↑ A. Kahan, The Plow, the Hammer, and the Knout: an economic history of eighteenth century Russia (Univ. of Chicago Press, 1985)
- ↑ Norman Mutton, 'The marked bar association: price regulation in the Black Country wrought iron trade' West Midland Studies 9 (1976), 2-8.
- ↑ On the Corrosion Resistance of the Delhi Iron Pillar, R. Balasubramaniam, Corrosion Science, Volume 42 (2000) pp. 2103–2129
- ↑ On the Growth Kinetics of the Protective Passive Film of the Delhi Iron Pillar, R Balasubramaniam, Current Science, Volume 82 (2002) pp. 1357–1365
- ↑ On the Origin of High Phosphorus Content in Ancient Indian Iron, Vikas Kumar and R. Balasubramaniam, International Journal of Metals, Materials and Processes, Volume 14 (2002) pp. 1–14
- ↑ Martha Goodaway, 'Phosphorus in antique iron music wire' Science 236 (1987) 927–32.
- ↑ A. Lucas, Wind, Water, Work: Ancient and Medieval Milling Technology (Brill, Leiden NL and Boston Mass. 2006), 251–5 347.
- ↑ 16.0 16.1 R. F. Tylecote, A History of Metallurgy (2nd edn, Institute of Metals 1992), 46–57 62–66.
- ↑ R. F. Tylecote, A History of Metallurgy, 75–76.
- ↑ R. F. Tylecote, A History of Metallurgy, 100–1.
- ↑ Richard Pococke The travels through England ... during 1750, 1751, and later years, ed. J.J. Cartwright (Camden Soc. n.s. 42, 1888), 13; W. Lewis, 'The Chemical and Mineral History of Iron' (MS in Cardiff Central Library, c. 1775) iv, 76
- ↑ G.C. Pollard, 'Experimentation in 19th-century bloomery iron production: Evidence from the Adirondacks of New York' Historical Metallurgy 32(1) (1998), 33–40.
- ↑ H. R. Schubert, History of the British Iron and Steel Industry from 450 BC to AD 1775 (Routledge and Kegan Paul, London 1957), 299–304.
- ↑ A. den Ouden, 'The production of wrought iron in Finery Hearths' Historical Metallurgy 15(2) (1981), 63–87 and 16(1) (1982), 29–32.
- ↑ G. R. Morton and N. Mutton, 'The Transition to Cort's Puddling Process' Journal of the Iron and Steel Institute 205 (1967), 723–4.
- ↑ R. Hayman, 'The Cranage brothers and eighteenth-century forge technology' Historical Metallurgy 28(2) (2004), 113–20.
- ↑ Morton and Mutton, 725–6.
- ↑ R. A. Mott (ed. P. Singer), Henry Cort, The Great Finer (The Metals Society, London 1983).
- ↑ 27.0 27.1 27.2 27.3 27.4 27.5 Rajput, R.K. (2000), Engineering Materials, S. Chand, p. 223, ISBN 8121919606
- ↑ W. K. V. Gale, The Iron and Steel Industry: a Dictionary of Terms (David and Charles, Newton Abbot 1971), 165.
- ↑ 29.0 29.1 Overman, Fredrick (1854), The Manufacture of Iron, in All Its Various Branches, Philadelphia: H. C. Baird, pp. 267, 287, 344, http://books.google.com/books?id=Gani2eHvhAkC
- ↑ R. F. Tylecote, 'Iron in the Industrial Revolution' in R. F. Tylecote, The Industrial Revolution in Metals (Institute of Metals, London 1991), 236–40.
- ↑ 31.0 31.1 31.2 31.3 31.4 31.5 31.6 31.7 31.8 Camp, James McIntyre; Francis, Charles Blaine (1920), The Making, Shaping and Treating of Steel, Pittsburgh: Carnegie Steel Company, pp. 173–174, http://books.google.com/books?id=P9MxAAAAMAAJ
- ↑ W. K. V. Gale, Dictionary, 137.
- ↑ W. K. V. Gale, The British Iron and Steel Industry (David and Charles, Newton Abbot, 1967), 79–88.
- ↑ G. Rydén, 'Responses to Coal Technology without Coal: Swedish Iron Making in the Nineteenth Century' in C. Evans and G. Rydén (eds.), The Industrial Revolution in Iron: The impact of British coal technology in 19th century Europe (Ashgate, Aldershot, 2005), 121–4; C. Evans and G. Rydén, Baltic Iron in the Atlantic World in the 18th century (Brill, Leiden NL and Boston, Mass.), 282–5.
- ↑ Stuart B Smith and W K V Gale, 'Wrought iron again: the Blists Hill Ironworks officially opened' Historical Metallurgy 21(1), 1987, pp. 44-5.
- ↑ About wrought iron, http://www.realwroughtiron.com/about_wrought_iron-217.html, retrieved 2008-02-22.
- ↑ Husband, Joseph; Harby, William (1911), Structural Engineering, New York: Longmans, Green, and Co., p. 21, http://books.google.com/books?id=pktDAAAAIAAJ, retrieved 2008-02-22.
- ↑ Byrne, Austin Thomas (1899), Inspection of the Materials and Workmanship Employed in Construction (1st ed.), New York: John Wiley & Sons, p. 105, http://books.google.com/books?id=2U80AAAAMAAJ, retrieved 2008-02-22.
- ↑ Scoffern, John (1861), The Useful Metals and Their Alloys, Including Mining Ventilation, Mining Jurisprudence, and Metallurgic Chemistry, London: Houlston and Wright, p. 328, http://books.google.com/books?id=SSkKAAAAIAAJ, retrieved 2008-02-20.
- ↑ Adams, Henry (1891), Handbook for Mechanical Engineers (2nd ed.), New York: E. & F. N. Spon, p. 29, http://books.google.com/books?id=0q03AAAAMAAJ, retrieved 2008-02-20.
- ↑ Hudson, J.C., 1931-43, Reports of the Corrosion Committee's Field Tests, Iron and Steel institute.
- ↑ 42.0 42.1 Dr. JP Chilton, 1929 - 2006, http://www.msm.cam.ac.uk/Department/Material_eyes/16/Chilton.html, retrieved 2008-11-29.
- ↑ 43.0 43.1 43.2 Walker VII, Robert (April 2002), "The Production, Microstructure, and Properties of Wrought Iron", Journal of Chemical Education 79 (4): 443–447, http://www.archaeometry.dk/Jern/Walker%20VII,%20Robert;%20The%20Production,%20Microstructure,%20and%20Properties%20of%20Wrought%20Iron.pdf.
- ↑ Chilton & Evens, Journal of the Iron and Steel Institute, 1955
- ↑ Harvey, L., The role of Slag Inclusions in the corrosion of wrought iron, dissertation University of Bradford, 1996
- ↑ Balasubramaniam, R (2003-01-25), "Delhi iron pillar and its relevance to modern technology", Current Science 84 (2): 162–163, http://www.iisc.ernet.in/currsci/jan252003/126.pdf.
- ↑ 47.0 47.1 47.2 47.3 47.4 Oberg, Erik; et. el. (2000), Machinery's Handbook (26th ed.), New York: Industrial Press, Inc., pp. 476, ISBN 0-8311-2666-3
- ↑ Smith, Carroll (1984), Engineer to Win, MotorBooks / MBI Publishing Company, pp. 53–54, ISBN 0879381868
- ↑ Solids and Metals - Specific Gravity, http://www.engineeringtoolbox.com/specific-gravity-solids-metals-d_293.html, retrieved 2008-02-20.
- ↑ Pole, William (1872), Iron as a Material of Construction: Being the Substance of a Course of Lectures Delivered at the Royal School of Naval Architecture, South Kensington (Revised and Enlarged ed.), London: E. & F. N. Spon, pp. 136–137, http://books.google.com/books?id=lwAKAAAAIAAJ&printsec=titlepage&source=gbs_summary_r, retrieved 2008-02-20.
- ↑ Richter, Victor von; Smith, Edgar Fahs (1885), A Text-book of Inorganic Chemistry (2nd ed.), Philadelphia: P. Blakiston, Son & Co., p. 396, http://books.google.com/books?id=7Q45AAAAMAAJ, retrieved 2008-02-21.
- ↑ American Technical Society (1916), Cyclopedia of Applied Electricity, 1, American Technical Society, p. 14, http://books.google.com/books?id=zaMOAAAAYAAJ&source=gbs_other_versions_sidebar_s&cad=6, retrieved 2008-02-21.
- ↑ Timbie, William Henry; Bush, Vannevar (1922), Principles of Electrical Engineering, New York: John Wiley & Sons, Inc., pp. 318–319, http://books.google.com/books?id=X6dEAAAAIAAJ, retrieved 2008-02-21.
- ↑ http://www.realwroughtiron.com/about_wrought_iron-217.html
Bibliography
- Bealer, Alex W. (1995), The Art of Blacksmithing, Edison, NJ: Castle Books, pp. 28–45, ISBN 0785803955